Difference between revisions of "AmmoniaSensor"
| Line 204: | Line 204: | ||
| 0.8 || || || 5264.67 || || || || || || || 4984.48 | | 0.8 || || || 5264.67 || || || || || || || 4984.48 | ||
|} | |} | ||
| + | |||
| + | =11 January 2023= | ||
Revision as of 00:49, 13 January 2023
We plan to make a simple ammonia sensor that make use of ammonia test kit. This test kit works by taking 5ml of water that you want to test and then mixed with the coloring agent that are given by the test kit. After mixing the water will develop a color which corresponds to the level of ammonia that the water had.
Contents
18 August 2022
We found a color sensor that had a potential for being used in this projects. TCS34725 sensor works by detecting red, green and blue color spectrum and intensity into signal. Based on the early testing that we got from this sensor, it was able to perceive the color difference on an object but the accuracy of the colors are dependent on how much the reflection of color could come to the sensors.
Testing Result
Using the current sensor we are going to test it directly with a sample water containing ammonia in various level from 0-8 ppm that are set to follow the color level chart from the test kit. The water is put in a clear 5ml container so we could shine light through the container to get the proper color reading.
From the experiment, we found that we could get a better reading if we used external light source than using the build in one as the source of light is too close towards the sensor which could affect the reading. We used the flashlight of our phone as our source of light and put the 5ml container above the light and have it glow through it from the bottom of the container.
From the testing result we got the following value as seen in the graph below.
10 October 2022
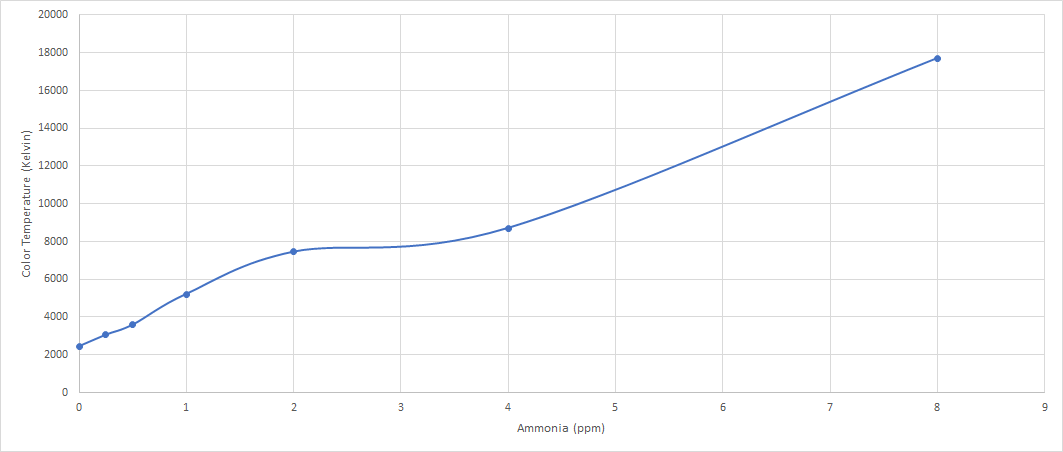
Testing for ammonia at different level concentration of Ammonia from 1- 8 ppm. Redoing the test since there is a possibility of a wrong concentration that are used in the previous test.
| PPM | Colour Temp | SD |
|---|---|---|
| 1 | 4525 | 4.01 |
| 2 | 5624 | 15.83 |
| 3 | 7514 | 87.40 |
| 4 | 9902 | 146.18 |
| 5 | 11607 | 65.38 |
| 6 | 12787 | 485.55 |
| 7 | 16072 | 131.30 |
| 8 | 17669 | 456.84 |
11 October 2022
Testing the ammonia level below from 0 – 1 ppm at various level of concentration
| PPM | Colour Temp | SD |
|---|---|---|
| 0 | 2588 | 3.39 |
| 0.2 | 2969 | 19.90 |
| 0.4 | 3217 | 27.75 |
| 0.5 | 3491 | 6.03 |
| 0.6 | 3601 | 13.42 |
| 0.8 | 3883 | 9.88 |
| 1.0 | 4573 | 54.60 |
All test that we have done so far are done with DI water. Next step we will try using water with some turbidity and see if there's a significant difference on the result.
25 October 2022
Make a new prototype case for the ammonia sensor For now the case still use a phone as a light source. The next iteration will integrade the case with an LED light source.
22 November 2022
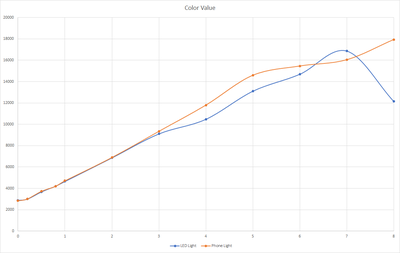
Done testing the sensor with lake water as our new base for dilution. With this new test we also tried a new LED light that we got that could operate from 2.7V - 3V. During the testing we will also want to know if this new light source have a significant impact to the reading. For the first test we set the LED at 2.7V and the result are shown below.
| PPM | LED Light | Phone Light |
|---|---|---|
| -1* | 6022.05 | 5960.86 |
| 0 | 2886.62 | 2843.14 |
| 0.2 | 2998 | 3026.10 |
| 0.5 | 3672.67 | 3737.43 |
| 0.8 | 4209.57 | 4206.05 |
| 1 | 4648.29 | 4714.81 |
| 2 | 6856.10 | 6897.10 |
| 3 | 9102.57 | 939347.24 |
| 4 | 10470.10 | 11794.30 |
| 5 | 13100.2 | 14585 |
| 6 | 14688.6 | 15453.7 |
| 7 | 16866.1 | 16039.2 |
| 8 | 12158.1 | 17927.5 |
Based on the results, the new LED light gave almost the same result from 0 - 4 ppm compared when we used the original phone for the light source. Above that level, the sensor gave a lower reading and goes weird at 7-8 ppm. This might be affected by the amount of lights that are captured by the sensor as the higher ppm solution have a darker color and it absorbs most of the light that are coming out from the led light, reducing the sensor ability to read the correct value.
5 December 2022
Turbidity test on ammonia solution Test are done on by making a 0.4 ppm solution on a 50ml container by dilution. The dilution are done by DI water that are mixed with various value of mud to replicate the effect of turbidity on various level.
One thing that we done wrong on this level is having the turbid water mixed outside of the 50 ml bottle hence, we haven't actually validate if the concentration of the ammonia is the same on all container.
But preliminary result from the testing shows that turbidity does affect the reading as the color of the liquid goes more towards yellow with the increase in turbidity.
| Ammonia Test | ||||
|---|---|---|---|---|
| NTU | MIN | AVG | MAX | STD DEV |
| 0 | 4672 | 4688.33 | 4708 | 10.85 |
| 17.2 | 3570 | 3581.29 | 3593 | 7.22 |
| 33.6 | 3485 | 3491.24 | 3498 | 3.46 |
| 111 | 3229 | 3239.67 | 3252 | 6.89 |
| 140 | 3061 | 3074.71 | 3086 | 8.10 |
9 December 2022
The main point of the testing is to capture the relationship between turbidity and ammonia concentration in the reading. Since turbidity may have a variance in between subsample that are taken. To ensure the same level of turbidity the test are done by first ensuring the subsample of the turbidity liquid are in the acceptable range. steps taken are as follow :
- Shake the main sample turbidity bottle
- take half of the turbidity liquid into the sampling bottle (7ml/15ml)
- Re-shake the main sample turbidity bottle
- fill the other half into the sampling bottle
- shake the sampling bottle and do a turbidity measurement on the subsample.
- repeat step 1- 5 until we have 5 sub sample
- Start dosing ammonia into the subsample.
Results are as follow:
| PPM | NTU | 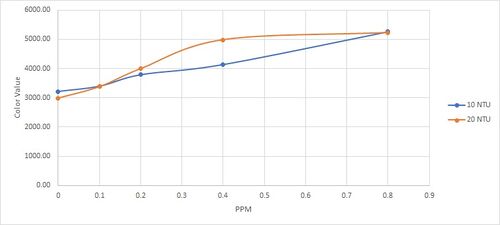
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | ||||||||||
| 7.71 | 7.78 | 7.94 | 8.27 | 9.45 | 22.2 | 23.3 | 24.6 | 25.3 | 25.7 | ||
| -1 | 6920.86 | 6617.29 | |||||||||
| 0 | 3218.76 | 2992.29 | |||||||||
| 0.1 | 3399.00 | 3382.48 | |||||||||
| 0.2 | 3792.38 | 3996.86 | |||||||||
| 0.4 | 4140.86 | 4984.48 | |||||||||
| 0.8 | 5264.67 | 4984.48 | |||||||||